UF MSE researchers have developed an injectable composite that spontaneously forms into nanofibers resembling the microscopic structure of the human body and could ultimately be custom designed for an infinite number of biomedical applications including connective-tissue repair, drug delivery, wound healing or skin care.
The discovery came while evaluating a synthetic polymer substrate as a potential cell-growth platform. The cells were performing modestly on the new surface, but to get them to both attach to the synthetic material and actually thrive they typically need at least one of a special set of proteins to also be present, namely fibronectin, laminin or collagen.
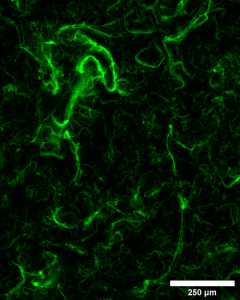
Building upon some of their earlier work, Allen Research Group leader, Josephine Allen, Ph.D., and NIH Predoctoral Fellow Bryan James, a Ph.D. candidate, thought to combine DNA aptamers with collagen. Aptamers are strands of DNA or RNA that recognize and bind to specific targets, similar to how a key fits a lock. They can bind to proteins, live cells or even other molecules. Most notably, these little strands of DNA can also activate cells and promote cellular responses.
It turned out that when the DNA aptamer and collagen were mixed together at specific ratios, nanofibers formed between them nearly instantaneously and, more notably, all on their own. Plus, the cells seemed to like the nanofibers, as well.
“We saw the cells preferentially interact with the fibers in a manner similar to how they behave in the body,” Dr. Allen said.
James said, “Initially, we weren’t sure what the fibers were, and thought it might be sample contamination. But after replicating the experiment several times we found that these fibers always formed at a distinct ratio of 25% collagen : 75% aptamer. We felt we were definitely onto something.”
With this new insight, the team, led by Dr. Allen and composed of James, junior Biomedical Engineering (BME) major Sophia Saenz, sophomore BME major Paxton Guerin and Cypress Bay High School (Weston, Florida) senior Anastacia van Gent began investigating the phenomena as a possible avenue for engineering bone tissue. Could these nanofibers be mineralized and support bone cell growth? Are they also capable of promoting angiogenesis (blood vessel formation)? The findings so far suggest both theories to be true, and each study is currently being assessed for publication in two biomaterials journals. Their initial findings have already been published in ACS Biomaterials Science & Engineering.
Dr. Allen and James are particularly excited about the simplicity and rapidity for synthesizing these materials.
“The synthesis conditions are simple and with a synthesis time of literally seconds to minutes, it opens the possibility of generating a lot of useful data in a short period of time, which ultimately advances the future utility of this technology,” Dr. Allen said.
In addition to the condensed timeline for synthesis, there are also a number of other advantages associated with this breakthrough. Its customization potential for different applications lies in the ability to modify the aptamer design and/or the type of collagen utilized. It also remains stable over long periods of time in wet or dry conditions, and because it’s a composite of natural biomolecules (DNA and collagen), it reduces potential toxicity for both cosmetic and biomedical applications.

